Betaine and its mode of action
What is betaine?
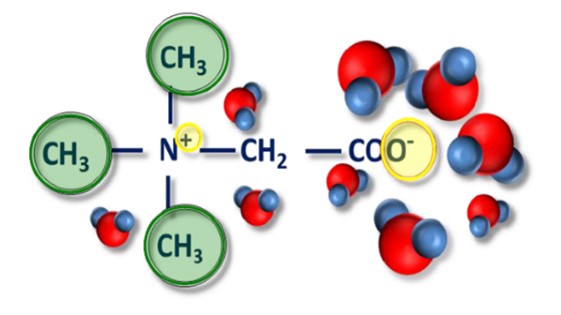
It was first discovered in the juice of sugar beets. Naturally accumulated in plants as osmolytes to protect against salt and temperature stress. Derivative of glycine (amino acid). A neutral molecule with a bipolar structure (zwitterion) as shown in Fig. 1 contains three methyl groups.
-
Betaine functions as(mode of action)
Methyl donor
Methyl groups used for protein synthesis and other metabolic processes. Methyl groups play a pivotal role in several cellular processes, including DNA methylation, synthesis of phosphatidylcholine, and protein synthesis. Choline and betaine are both capable of donating methyl groups. However, for choline to do so, it must first be converted into betaine as shown in Fig. 2. In poultry, the capacity to synthesize betaine from choline is limited, thus making dietary supplementation the primary source.

Betaine can substitute for choline in performing the following functions:
1) Regulating fat metabolism in the liver to prevent abnormal fat accumulation in hepatocytes.
2) Serving as a methyl donor for the formation of methionine and creatine, through its involvement in the transmethylation pathway.
Betaine cannot replace choline in the function of maintaining cell membrane and structure as an emulsifier to transport lipids, since choline is a constituent of phospholipids. Similarly, betaine cannot replace choline as a precursor of acetylcholine in the transmission of nerve impulses.
Osmo-regulator
The ability to bind and retain water in a reversible manner.Osmolytes are compounds that aid in the regulation of osmotic pressure within cells and tissues, playing a crucial role in preserving cellular integrity. Dehydration, disease, heat stress, and other factors can cause alterations in the water content of cells. Osmolytes can be either inorganic ions such as Na+, K+, and Cl-, or organic compounds such as amino acids, certain sugars, and betaine. Betaine plays a crucial role in stabilizing cellular metabolic function during periods of stress, preserving the cell's capacity to uptake nutrients, unlike osmolytes such as Na+, K+, and Cl-. Moreover, it offers protection to intracellular enzymes against osmotic inactivation.